
Microtextured Bioresorbable Protective Sheet
Indicated for Open and Laparoscopic Procedures
MAST® Biosurgery Resorbable PLA Film Technology
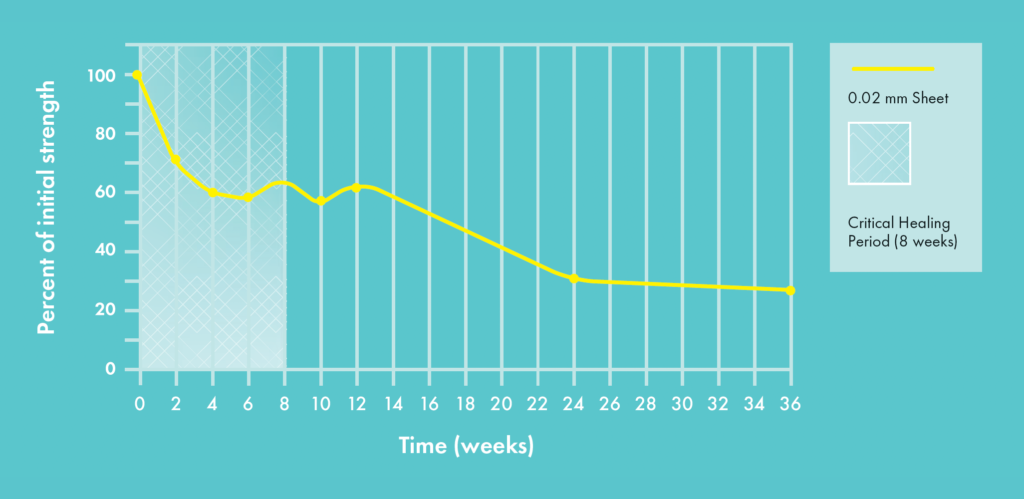
The SurgiWrap® FROSTA™ resorbable protective film retains more than 80% of polymer integrity throughout the critical healing period
SurgiWrap® Bioresorbable Sheet polymer material is fabricated from the same lactic acid molecular building blocks that occur naturally in the human body. The polymers, which result from lactic acid derivatives, are generally referred to as PLA or polylactide. The SurgiWrap® Bioresorbable Sheet is made from an amorphous bioresorbable copolymer of 70:30 Poly(Llactide-co-D,L-lactide).
The degradation of polylactide polymer (PLA) occurs in two phases:
- Water penetrates the implant and severs the polymer chains into smaller units
- Individual lactide molecules are metabolized in the liver into CO2 and H2O and excreted or exhaled
Barrier Applications Using Resorbable Protective Film
MAST® WetGrip MicroSurface™ Technology
“The wetter the better” – L. Bluecher, Inventor

Surgeon Approved
SurgiWrap® Bioresorbable Sheet was FDA cleared in 2003
SurgiWrap® FROST™ Bioresorbable Sheet was recently FDA cleared in 2021
MAST® Biosurgery PLA Resorbable Protective Films are clinically proven to be Safe and Effective in support of surgical repairs of soft tissues
SurgiWrap® FROST™ is fabricated using the SurgiWrap® Bioresorbable Sheet PLA film technology
SurgiWrap® FROST™ features WetGrip™ MicroSurface to assist with intraoperative positioning, re-positioning and fixation
The WetGrip™ MicroSurface is limited to a single-side of the film and does not adhere to tissue; it clings to tissue using the inherent moisture on tissue to create a benign and “magnet like” attachment to the surgical site
SurgiWrap® FROST™ is a resorbable protective film that is designed to temporarily isolate surgical repairs from the adjacent organs through the first few weeks of wound healing to reduce potential for unintended soft tissue attachments
Indications:
The SurgiWrap® FROST™ Bioresorbable Protective Sheet is to be used wherever temporary wound support is required, to reinforce soft tissues where weakness exists or for the repair of hernia or other fascial defects that require the addition of a reinforcing or bridging material to obtain the desired surgical result. This includes, but is not limited to the following procedures: colon and rectal prolapse. The Resorbable Protective Film minimizes tissue attachment to the device in case of direct contact with the viscera. The device is indicated for open and laparoscopic procedures.
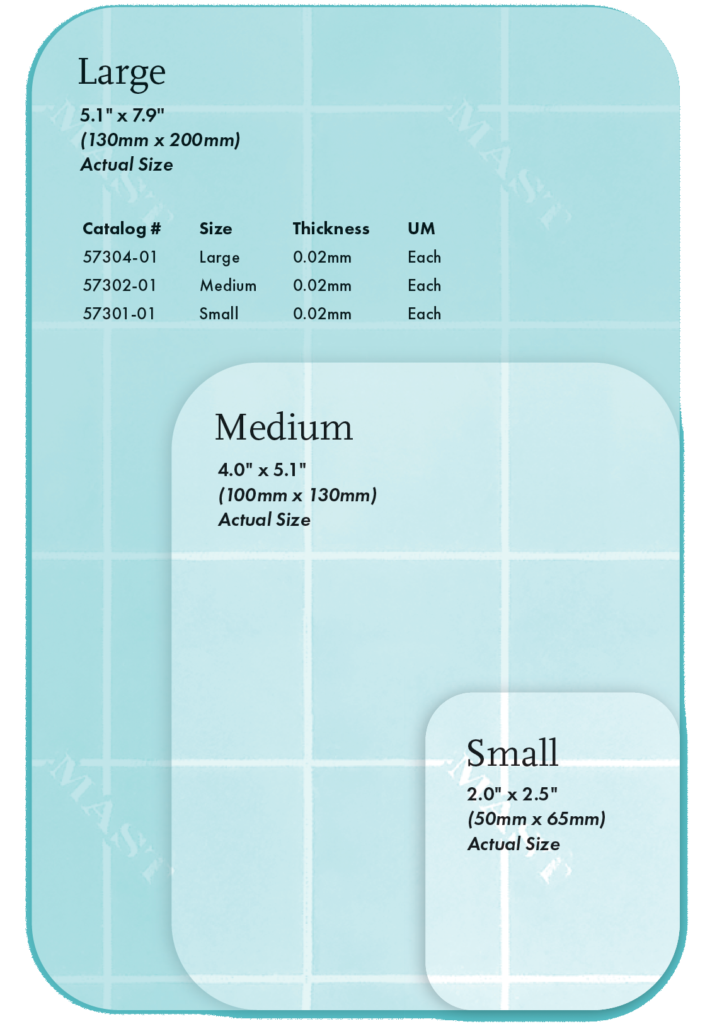
Ordering Information
US Patents: 10, 130, 459 10,292, 806 10, 433, 924 10, 507, 015 10, 758, 380 • International Patents Pending
All trademarks and registered trademarks are the property of the irrespective owners.
Caution: Federal Law Restricts this Device to sale by or on the order of a Physician
